Chem Lecture 3 – Limiting Reactants, Percent Yield, Molar Mass, Percent Mass, Molarity – Flashcards
Unlock all answers in this set
Unlock answersquestion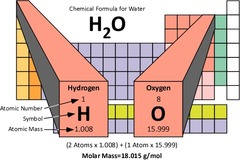
molar mass

answer
the mass of one mole of a substance -read off the periodic table
question
Molar mass of molecules
answer
sum of all the atomic masses
question
mass percent

answer
mass of component/total mass
question
empirical formula
answer
simplest formula, lowest whole number of moles --relative number of atoms Hydrogen peroxide H202(molecular formula)-> H0 (empirical formula)
question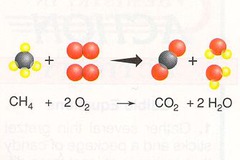
combustion

answer
burning of a substance with oxygen all carbons go to CO2 all hydrogens go to water
question
stoichiometry
answer
relationship between quantities of reactants and products
question
2Mg + O2 -> 2MgO How many moles MgO can be produced from 1.5moles of O2?
answer
(1.5molesO2 x 2molesMgO) / 1 mole O2 = 4=3 moles MgO
question
2Mg + O2 -> 2MgO How many grams of Mg are needed to produce 2.25 moles MgO
answer
(2.25molesMgOx 2mole Mg)/ 2molesMgO) x 24.305gMg = 54.7 g Mg
question
2Mg + O2 -> 2MgO How many moles of Mg are needed to completely react with 1.5gO2
answer
(1.5gO2x1moleO2)/32.00gO2 x (2molMg/1molO2) = 0.094mol Mg
question
Limiting reactants
answer
the one that would produce least product 1. find moles of reactants (if in grams go to moles) 2. use mole ration to find moles of product each could make
question
Excess (leftover) reactant
answer
Original amount - amount used -use mole ration how much leftover reactant was used
question
theoretical yield
answer
max amount of product the limiting reactant can produce
question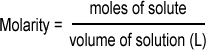
molarity, M

answer
concentraton unit moles solute/liters of solution
question
moles
answer
MxV (in liters) molarityxvolume
question
percent yield
answer
actual yield/theoretical yield



