Organic Chemistry Ch 17 – Flashcards
Unlock all answers in this set
Unlock answersquestion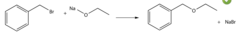
Choose an equation describing a way in which benzyl ethyl ether could be prepared by a Williamson ether synthesis.
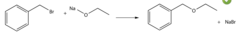
answer
Benzyl bromide + sodium ethoxide= benzyl ethyl ether
question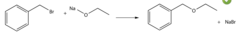
Choose an equation describing a way in which benzyl ethyl ether could be prepared by a Williamson ether synthesis.
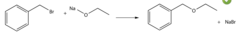
answer
Sodium benzyl oxide + bromoethane= benzyl ethyl ether
question
What is a Williamson reaction and what does it react best with?
answer
1. Sn2 reaction for synthesizing ethers 2.Works best for methyl and primary alkyl halides
question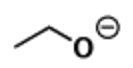
Draw an alkoxide and define it
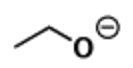
answer
Conjugate base of alcohol
question
What is OsO4 (osmium tetroxide) used for?
answer
It is used to convert alkenes into vicinal diols
question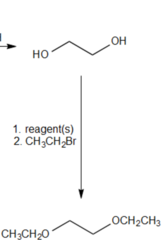
What reagent would you use to convert 1,2-ethanediol and bromoethane into 1,2-diethoxyethane?

answer
Sodium
question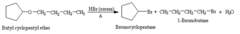
A dialkyl ether was allowed to react with excess hydrogen bromide, giving a mixture of bromocyclopentane and 1?bromobutane. Identify the ether.
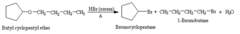
answer
Butyl cyclopentyl ether
question
When allowed to react with excess hydrogen bromide, an ether gave only one benzyl bromide. What was the ether?

answer
Dibenzyl ether
question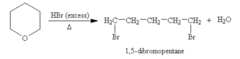
When allowed to react with excess hydrogen bromide, an ether gave one mole of 1,5-dibromopentane per mole of ether. What was the ether?
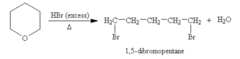
answer
Oxane (aka tetrahydropyran, aka oxacyclohexane)
question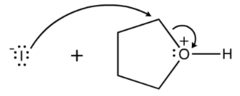
Draw the reaction between Iodine ion and protonated tetrahydrofuran

answer
Iodine ion deprotonates the protonated tetrahydrofuran, causing the ring to open up
question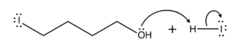
Draw the reaction between the 4-iodobutanol and the new HI molecule

answer
Lone pair on the O attracts the hydrogen from the HI, I becomes an Ion once more
question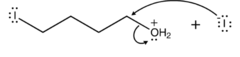
Draw the reaction between the protonated 4-iodobutanol and the Iodine ion

answer
The protonated 4-iodobutanol now has water as a leaving group. Water boots off and the Iodine ion swoops in to takes its place, giving us 1,4-diiodobutane
question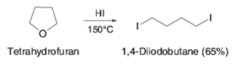
What product is synthesized from the reaction of tetrahydrofuran with HI and heat?

answer
1,4-diiodobutane
question
What reagent reacts with propene to produce epoxypropane?

answer
CH3CO3H
question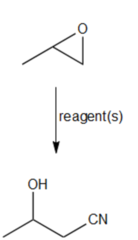
What reagent reacts with epoxypropane to produce a cyanoalcohol?

answer
1. NaCN, (DMSO ) or CH3S(O)CH3 2. H2O
question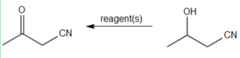
What reagent reacts with a cyanoalcohol to form 1-cyano-2-propanone?

answer
(Pyridinium chlorochromate) PCC, CH2Cl2
question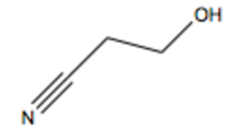
What is the principal organic product formed in the reaction of ethylene oxide with sodium cyanide (NaCN) in aqueous ethanol?

answer
Anionic nucleophil NaCN opens the epoxide which is then protonated to form an alcohol
question
What is the principal organic product formed in the reaction of ethylene oxide with phenyllithium (C6H5Li) in diethyl ether, followed by addition of dilute sulfuric acid?

answer
Phenyllithium provides a phenyl group as a nucleophil which attacks the epoxide, ethylene oxide, which opens the ring to form 2-phenylethanol
question
What is the principal organic product formed in the reaction of ethylene oxide with sodium azide (NaN3) in aqueous ethanol

answer
Sodium azide provides azide as a nucleophile which attacks ethylene oxide and opens the ring to form 2-azidoethanol
question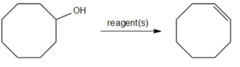
What reagent can convert cyclooctanol into cyclooctene?
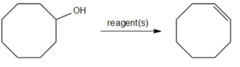
answer
H2SO4 and heat cause dehydration of an alcohol to yield an alkene
question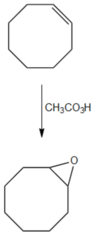
What reagent converts cyclooctene into cyclooctene oxide?

answer
CH3CO3H (peracid) convert alkenes into epoxides in one step
question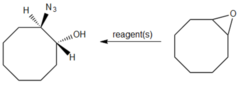
What reagent converts cyclooctene oxide into trans-2-azidocyclooctanol?

answer
1. NaN3, DMSO 2. H2O Sodium azide (NaN3) in DMSO can open the epoxide to give a trans-configured alcohol after subsequent exposure to H2O
question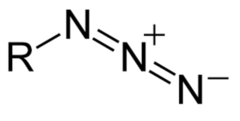
Draw an azide (what is an azide?)
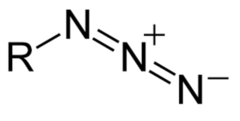
answer
Triple nitrogens bound together with double bonds
question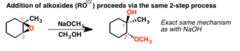
Describe the reaction between an epoxide and sodium methoxide in methanol

answer
Sodium methoxide acts as a base. Nucleophilic attack to the epoxide at the least substituted position produces an Sn2 reaction to produce a vicinal diol where the epoxide was
question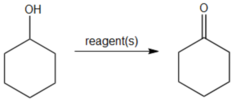
What reagent could you use to convert cyclohexanol to cyclohexanone?

answer
Na2Cr2O7, H2SO4, H2O Sodium dichromate in aqueous acid will convert secondary alcohols to ketones. Could also use PCC
question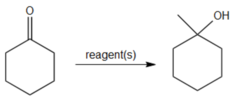
What reagent could you use to convert cyclohexanone to 1-methylcyclohexanol?

answer
1. Ch3MgBr, ether 2. Water Grignard rxn using methylmagnesium bromide will produce 1-methylcyclohexanol in one step
question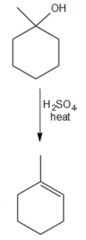
What reagent could you use to convert 1-methylcyclohexanol to 1-methylcyclohexene?

answer
H2SO4 and heat Sulfuric acid and heat will cause dehydration of the alcohol to produce an alkene
question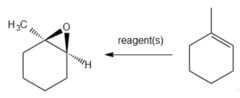
What reagent would you use to convert 1-methylcyclohexene to an epoxide?

answer
CH3CO3H Peracetic acid causes epoxide formation from alkenes
question
If you wanted to make an epoxide from an alkene what reagent would you use?
answer
Peracetic acid (CH3CO3H)
question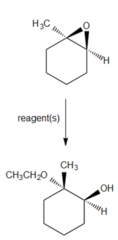
If you wanted to convert the epoxide into 2-ethoxy-2-methylcyclohexanol

answer
H2SO4(catalytic) and CH3CH2OH Ethanol and sulfuric acid will open the epoxide in a trans fashion, with ethanol bonding to the more highly substituted carbon of the epoxide
question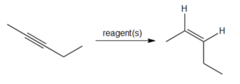
What would you use to convert 2-pentyne to cis-2-pentene?

answer
H2, Lindlar catalyst
question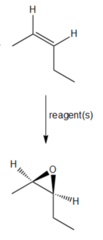
What would you use to convert cis-2-pentene into a cis-epoxide?

answer
CH3CO3H peracetic acids form epoxides out of alkenes
question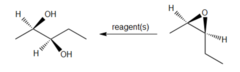
What would you use to convert a cis-epoxide into an (R,R)-2,3-pentanediol

answer
H2O, H2SO4 Aqueous acid will open the epoxide to form a vicinal diol in an anti fashion
question
What does H2SO4 in methanol do to an epoxide?
answer
Opens it up
question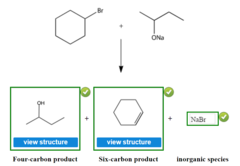
What products are formed when you add bromocyclohexane and sodium 2-butanolate

answer
1. butan-2-ol 2. 1-cyclohexene 3. NaBr
question
What happens when you combine iodoethane with sodium alkoxide?

answer
Sodium alkoxide acts as a nucelophile towards iodoethane to yield an alkyl ethyl ether
question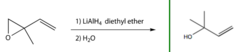
What happens when you add LiAlH4, diethyl ether and water to 3,4-Epoxy-3-methyl-1-butene?

answer
Lithium aluminium hydride reduces epoxides to alcohols and hydride is transferred to the less substituted carbon of the epoxide ring. The alkene double bond stays put.
question
What does LiAlH4 do to an epoxide?
answer
Reduces it to an alcohol



